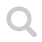Ammonia is a colorless gas with an unpleasant smell. The NH₃ molecule has a pyramidal form with triangular sides. Nitrogen is more electronegative than hydrogen which makes the molecule polar.
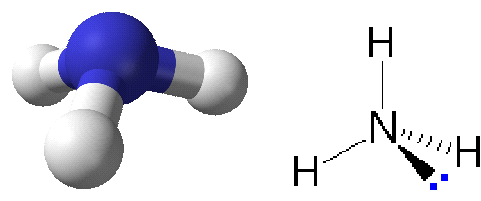
Two variants of presenting ammonia. Three hydrogen atoms are bound to a nitrogen atom in the NH₃ molecule. A nonshared electron pair "lone pair" is shown to the right, giving the electron arrangement around nitrogen a nearly tetrahedral symmetry.
Ammonia is used as refrigerant in industries or store freezer cases. The gas is also a common chemical in chemical factories for producing fertilizers, nitric acid and other nitrogen containing compounds. Dry ammonia is flammable in contact with oxygen.
Ammonia is a common weak base for instance used in household cleaning solutions. The gas is easily dissolved in water, forming an alkaline solution according the equilibrium reaction:
NH₃ + H₂O ↔ NH₄⁺ + OH⁻
Therefore ammonia is dangerous for the lungs and causes irritated eyes or skin.
Ammonia gas is known to show a strong absorption at 0.8 cm⁻¹ or 12.5 mm wavelength in the microwave region. When the NH₃ molecule is excited, once every 20.9 μs (microseconds) the nitrogen atom passes through the base forming a pyramid at the other side and 20.9 μs later it returns to its original position. This vibration back and forth has a frequency of 23,870 MHz and is denoted the ammonia resonator.
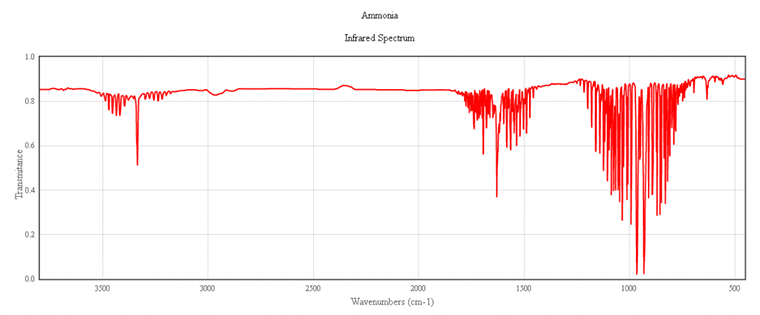
IR spectrum of ammonia, NH₃
The characteristic peaks in the IR spectrum of ammonia come from the N-H bonds. At 3400 cm⁻¹ (2.9 μm) the absorption is due to N-H strechings. The strong peaks at 1800 (5.6 μm) and 1000 cm⁻¹ (10 μm) are caused by H-N-H scissoring and N-H wagging, respectively.
References:
http://webbook.nist.gov/cgi/cbook.cgi?ID=C7664417&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC