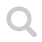Dinitrogen monoxide (nitrous oxide or laughing gas) is an uncolored gas with a sweetish smell. It is inert and non-flammable at room temperature, due to resonance which stabilizes the linear molecule.
N₂O, dinitrogen oxide, with an electron resonance structure.
High levels of N₂O can cause dizziness, euphoria or other symptoms such as vitamin B₁₂ deficiencey.
N₂O emissions are mainly due to automotive exhausts or to disintegration of biological material in manure or compost handling. Roughly one-third of the emissions are manmade, with agriculture the largest source. Since N₂O is a very powerful greenhouse gas it should not come out in the atmosphere - dinitrogen oxide is about 300 times stronger as a greenhouse gas than carbon dioxide, when their Global Warming Potentials (GWP) are compared.
N₂O is also involved in the upper atmosphere chemistry, breaking ozone molecules. Some molecules will sustain when they are transported to the upper atmosphere and eventually form radicals which destroy ozone. A thinner ozone layer in the stratosphere will transmit dangerous ultraviolet radiation to the earth, causing skin cancer and other health problems for living beings. Dinitrogen oxide has about the same ozone depleting potential (ODP) as some of the wellknown freons. ODP is a measure of stratospheric ozone destroyed by a chemical relative to the amount destroyed by the most harmful freon CFC-11, trichlorofluoromethane.
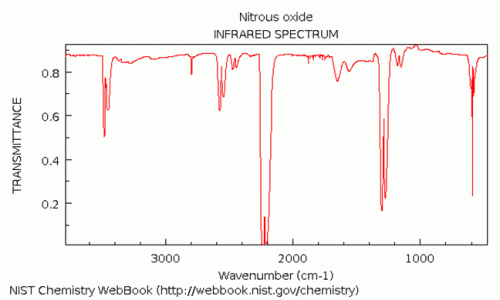
IR spectrum dinitrogen oxide
The largest IR stretching absorption for N₂O (dinitrogen oxide or nitrous oxide) is found at approximately 2200 cm⁻¹ and 1300 cm⁻¹, corresponding to a wavelength of about 4.5 μm and 7.7 μm, respectively. The latter peak might be interfered by methane, water and many other compounds. The peak at 4.5 μm is very close to an absorption peak of CO₂ - this problem can partly be eliminated by using a narrow band IR filter.