Methanal or formaldehyde is a gas at room temperature with a typical sharp smell. The chemical properties are influenced by the C=O group.
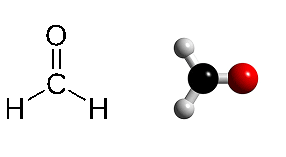
Methanal (formaldehyde, CH₂O) structure in two variants. The carbon atom is surrounded by two hydrogen and one oxygen atom in this planar molecule.
Methanal is poisonous at low levels and causes irritated eyes if the concentration is above 0.1 ppm. It is also extremely allergenic, which is problematic since it is common in construction materials such as foam insulation and plywood. Methanal is classified as a probable human carcinogen, perhaps causing leukemia according recent studies. Besides in construction materials, methanal is used as a precursor in chemical industry, disinfectant, biocide and as a reagent. The most characteristic peaks in the IR spectrum of methanal come from C-H and C=O bonds.
IR spectrum of methanal, CH₂O
The strongest peaks in the IR spectrum of methanal (formaldehyde) comes from C-H stretching at 2900 cm⁻¹ and C=O at 1750 cm⁻¹, corresponding to 3.4 μm and 5.7 μm, respectively. In addition, there are some weaker H-C-H peaks due to stretching, scissoring, rocking and wagging, see the spectrum and molecule drawings below.
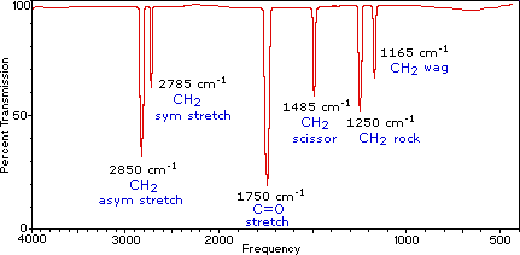
The IR spectrum of methanal or formaldehyde. It is copied from tutorial pages by Department of Chemistry at Michigan State University, USA. Read more
IR active vibrations of methanal. Vibration 1 is called stretching, 2 wagging, 3 scissoring, 4 symmetric stretching, 5 rocking and 6 is called asymmetric stretching.