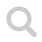The water molecule is bent with an angle of ~104° and it is polar because the oxygen is negative and both the hydrogen atoms are slightly positive.
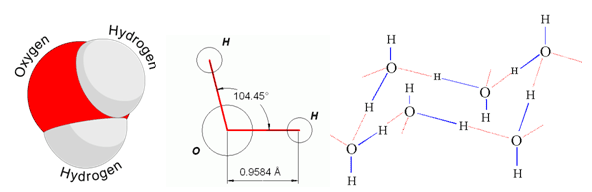
Structure of water molecules showing the important H-O-H angle. The bond distance is near 1 Å, which means 1*10⁻¹⁰ m. A structure model of ice is outlined to the right.
Water is an uncolored, non-smelling and non-toxic chemical. Water is causing the hydrological cycle on earth, where it is transforming between solid in ice/snow, liquid in lakes, oceans and rivers and vapor in air and clouds. Humidity can only be found on the lowest atmospheric levels, in the troposphere from 0 to max 16 km above sea level, where the concentration of H₂O water vapor is 1 - 4 volume %. Higher up, water molceules are decomposed into various radicals by sunlight, forming for example - OH, hydroxyl radical. Water is contributing to about 70% of the most powerful greenhouse gases when the sky is cloudy and slightly less at clear sky conditions. Dumping greenhouse gases into the atmosphere makes the air warmer and more humid. And since water vapor is itself a greenhouse gas, the increase in humidity amplifies the warming from carbon dioxide and other potent greenhouse gases. Increasing water vapour leads to warmer temperatures, which causes more water vapour to be evaporated - it is deeply worrying that warming and water IR absorption both increase in a spiraling cycle.
The IR spectrum of H₂O shows broad absorption at 3800 and 1600 cm⁻¹ (2.6 and 6.2 μm, respectively), due to O-H stretching and bending modes.
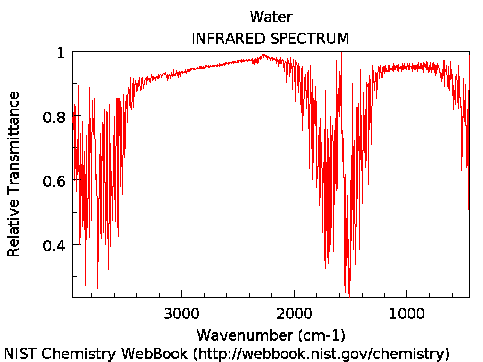
IR spectrum of water vapor, from NIST webpages. Absorption takes place where transmission goes down, as seen in the spectrum.
The peaks from water are not interfering with CO₂ but with many organic solvents such as ethanol as shown below:
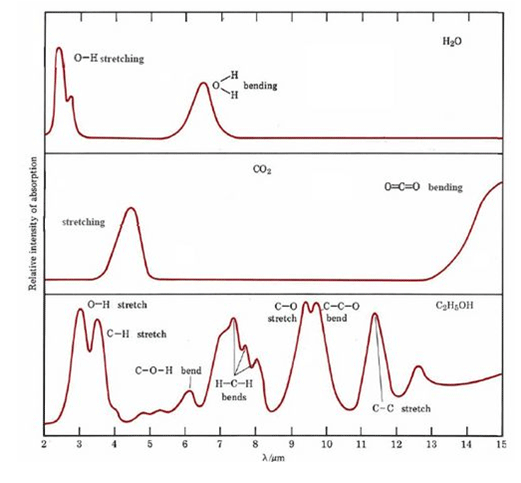
IR absorption spectra of three common chemicals, water, carbon dioxide and ethanol, taken from CoreChem at: http://wiki.chemprime.chemeddl.org/index.php/CoreChem:The_Spectra_of_Molecules:_Infrared