The freon gases are used as refrigerants and often abbreviated as R-11, R-40, aso. Another usage is as propellants in medical inhalants. Normally freon is inert at room temperature and they are more or less nontoxic for human beings. At a hot surface, more than 250℃, they might decompose to give toxic and corrosive HF(g) or other unpleasant gases.
The inertness of the freon gases creates a problem, as they survive in the environment for a long time, eventually diffusing into the stratosphere (altitudes of 25 - 40 km) to decompose and simultaneously destroy the UV radiation protective ozone layer. The freon with highest ozone depleting potential (ODP) is R-11 (CFC-11), trichlorofluoromethane. ODP is a measure of stratospheric ozone destroyed by a chemical relative to the amount destroyed by R-11. Besides freons there are other chemicals with high ODP, for example dinitrogen oxide and fire extinguishers such as halons.
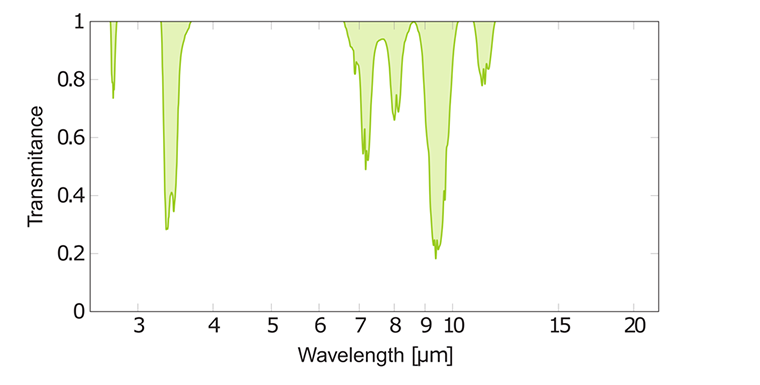
The IR spectra of various freon gases are rather complex. There are a number of peaks from stretching, rocking or deformation. The strongest C-F absorption peaks are often found on both sides of 10 μm, which is shown above for CFCl and CF₂Cl₂.
References:
http://webbook.nist.gov/cgi/cbook.cgi?ID=C75718&Type=IR-SPEC&Index=1#IR-SPEC
http://webbook.nist.gov/cgi/cbook.cgi?ID=C74986&Type=IR-SPEC&Index=1#IR-SPEC